The question of whether to continue authorizing glyphosate has been a central concern for the European Commission since 2017. This inquiry triggered a multifaceted and intricate debate involving scientific considerations, contradictory scientific studies, and pressures exerted by industrial lobbies. This controversy sheds light on the inherent economic dilemmas tied to this issue, directly impacting policymaking at the European level.
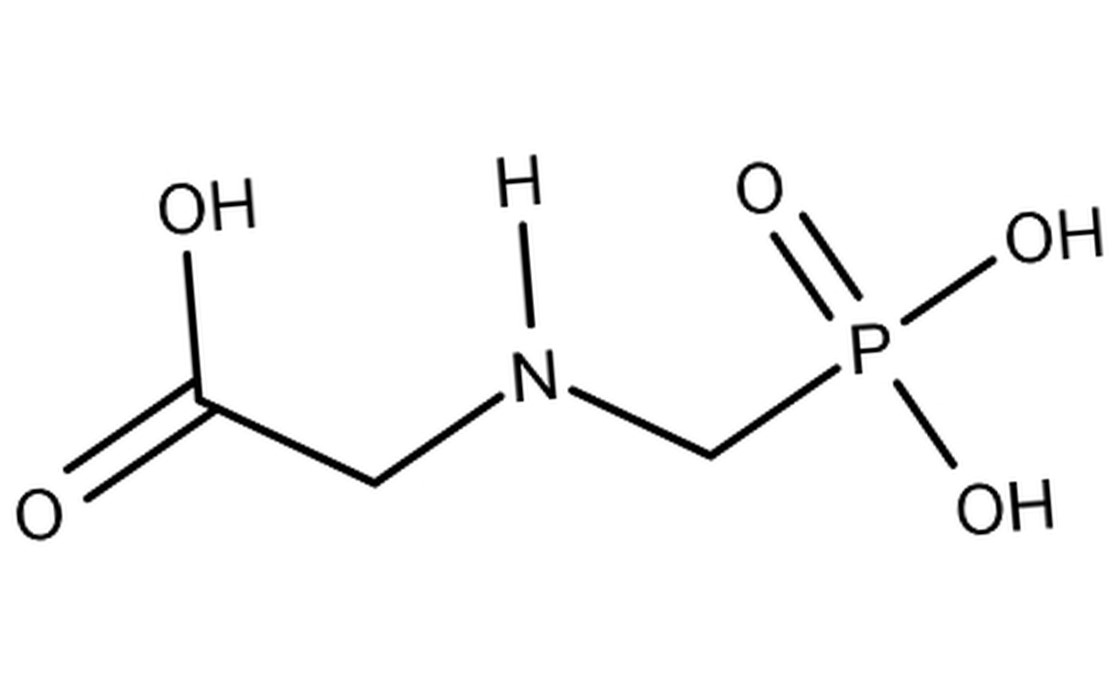
Glyphosate, an active ingredient present in numerous herbicides, has revolutionized agriculture since its introduction in the 1970s. Celebrated for its efficacy in weed control, it has become an indispensable tool for farmers due to its undeniable advantages. However, in 2015, a study by the International Agency for Research on Cancer (IARC), a branch of the World Health Organization (WHO), classified glyphosate as “probably carcinogenic to humans.” This assessment triggered growing concerns regarding its impact on human health, sparking a profound debate on its safety and suitability in modern agriculture.
This controversy has sparked intense discussions and contradictory assessments regarding glyphosate’s safety. It has also highlighted disparities in evaluations conducted by different international organizations, prompting heated debates on regulations and agricultural practices on a global scale. By exploring the nuances of this issue, we seek to understand how these aspects influence policymaking at the European level.
Science: Whom to Trust?
In the European Union, the approval granted to active substances used in pesticides is time-limited. As the current approval period nears its end, these substances undergo a renewal procedure. This process involves a comprehensive reassessment of the safety, efficacy, and environmental impact of the substance to decide whether to extend its market authorization. The primary goal is to ensure that the substance continues to meet stringent standards of safety and protection.
The evaluation process prior to the approval of active substances at the European level, such as glyphosate used in Roundup, is a scientific protocol involving several agencies. First, the European Food Safety Authority (EFSA), in cooperation with the competent authorities of EU member states, meticulously reviews all available scientific studies to provide advisory opinions to the European Commission. Subsequently, the Commission submits a proposal to the member states, allowing them to either ban or approve the substance, potentially accompanied by risk management recommendations. Member states then vote on this proposal.
However, the situation becomes significantly complicated concerning glyphosate due to conflicting assessments between the International Agency for Research on Cancer (IARC) and two complementary European agencies, namely the European Food Safety Authority (EFSA) and the European Chemicals Agency (ECHA). While the IARC classified glyphosate as probably carcinogenic in 2015, the EFSA, responsible for evaluating the risks associated with actual exposure to the substance, concluded that glyphosate was unlikely to be carcinogenic to humans the same year. The ECHA further supported this evaluation in 2017 and reiterated in 2022 that the scientific evidence available did not allow glyphosate to be classified as a carcinogen. ECHA nevertheless admits in this report that the substance causes serious eye damage and is toxic to aquatic life. This divergence of opinions among the agencies has added a layer of complexity to the controversy, making the procedure within the EU even more challenging.
This discrepancy partly stems from the evaluation methods used. Basic tests, such as those conducted by the EFSA, focus solely on the active substance, excluding other products present in the mixture used in agricultural fields. This initial divergence in results between different agencies raises significant questions about the evaluation methodology itself. Furthermore, research focusing on the combined substance, as used in real formulations, triggers debates; some researchers suggest that the presence of co-formulants might potentially increase glyphosate’s toxicity. Another critical aspect concerns the methodology followed within the EFSA itself. The European authority relies on data provided by companies producing these pesticides, which are neither publicly accessible nor subjected to external review. This lack of transparency raises concerns about the reliability of this information, leaving aside a portion of scientific literature in favor of industry analyses, thus generating distrust in these works. The EFSA’s final report, published on September 13, 2023, did not identify a “critical area of concern” for humans, animals, or the environment that would prevent glyphosate authorization. However, it highlighted “data gaps,” “unresolved issues,” and “long-term high risk for mammals in 12 out of 23 proposed glyphosate uses.”
The differences in opinions among international organizations regarding glyphosate’s safety stem from distinct evaluation methodologies, varying in study scope, data interpretation, and source transparency, resulting in divergent conclusions. While the IARC classified glyphosate as potentially carcinogenic, the EFSA and ECHA reached different conclusions, raising questions about how these studies are conducted and interpreted. These disparities raise concerns about the transparency and independence of evaluations, particularly when data originates from glyphosate-producing companies, potentially biasing scientific conclusions. Although controversial and not unanimously accepted, this report serves as the scientific basis upon which the European Commission based its proposal to renew glyphosate authorization until 2033, presented on September 19, 2023. As no majority was reached among EU member states on November 16, 2023, during the second vote on the European Commission’s proposal to renew the authorization of glyphosate, according to European rules, in the absence of an agreement, it was up to the Commission to decide, and it opted to approve glyphosate until 2033. This decision, made in a context where the EU maintains a strict pesticide authorization system with over 700 banned substances over 25 years, raises questions about the rigor of the evaluation and decision-making process.
Disinformation Campaign and Public Skepticism: The Monsanto Papers Case
Speaking of rigor, a new layer of scientific complexity lies not just in the methodology but in the legitimacy of the studies conducted. Monsanto’s lobbying and its implications in the decision-making process of the European Union, notably through the revelations of the “Monsanto Papers,” have shed harsh light on controversial practices and potential manipulations of scientific data related to glyphosate.
The publication of internal documents suggested that Monsanto might have been aware of the health risks associated with glyphosate long before these concerns were widely acknowledged publicly. More troublingly, they raised doubts about the transparency of scientific data presented to regulatory bodies, implying that the company could have concealed or downplayed certain harmful effects of glyphosate. The documents also hinted at potential excessive influence of Monsanto on studies and regulations concerning glyphosate, raising concerns about the independence of regulatory assessments. Then, the disclosure of thousands of documents in early 2017 exposed Monsanto’s lobbying practices aimed at influencing scientific reports. The multinational corporation sought to sow doubt in the public’s mind, denying any potential danger linked to glyphosate. It orchestrated the publication of content in mainstream and scientific media using a significant amount of literature authored by itself (ghostwriting), often without declaration of its involvement. This strategy also affected national control agencies, where scientists paid by Monsanto produced biased reports, altering the perception of the credibility of regulatory assessments. In other words, organized disinformation that influences scientific investigations and political positions.
Monsanto’s revealed lobbying practices, highlighted in the “Monsanto Papers,” raise serious doubts about regulatory integrity and study credibility, impacting public trust in regulatory decisions due to concerns about industry influence on scientific studies. The erosion of trust prompts doubts in regulatory objectivity, impacting public confidence, fostering a call for greater transparency and independence in regulatory scientific studies, emphasizing the need for unbiased, transparent research to ensure credibility in chemical safety decisions. These revelations have significantly undermined trust in the scientific studies used to assess the safety of glyphosate. They have questioned the integrity of regulatory approval processes, highlighting problematic connections between major agrochemical companies and the decisions made by regulatory authorities. Lawsuits lost by Monsanto in several countries, notably in the United States and France, have further strengthened public distrust in studies conducted by oversight agencies.
However, despite these revelations, trials against Bayer (former Monsanto) for cancer cases and public skepticism, there remain unresolved questions regarding the implementation of the precautionary principle in Europe. Why does this measure of caution and prevention not exert a stronger influence in the face of doubts about the safety of glyphosate, despite the evidence and challenges raised? This inquiry raises concerns about decision-making processes and the priority given to public health protection in current European policies.
Complex Response to a Complex Issue: The Application (Lack) of the Precautionary Principle in European Context
In accordance with European Union law (Regulation 1107/2009), Article 4 outlines the mandatory conditions that an active substance must fulfill to be approved for use in the European market. If these conditions are not met, an active substance cannot be (re)approved. Additionally, the precautionary principle, firmly established in EU law (Treaty on the Functioning of the European Union, Article 191), also underpins the EU pesticide regulation (Regulation 1107/2009, Article 1, paragraph 4), ensuring that “active substances or products placed on the market do not adversely affect human or animal health or the environment”. Therefore, if there is doubt regarding the potential harmful effects of an active substance or product on human or animal health or the environment, authorizations should not be granted.
In this context, the glyphosate case triggered the existing precautionary principle within the EU as early as 2015. Many activists base their arguments on this principle, asserting that in the absence of clear data and in the case of persistent doubt, the use of glyphosate should be subject to a ban. More than that, the ban on the use of the substance by private individuals in France, Belgium and other countries in the EU and the thousands of cases lost by Bayer (formerly Monsanto) should remove any doubt about the product’s dangerousness. However, the last ten years of decisions, the recent Council vote and the final decision by the Commission reflect an intricate situation. The issue arises from the purported impossibility of replacing glyphosate due to its cost-effectiveness and formidable efficacy. As so often, it’s a question of choosing between health and productivity, and the Commission chooses productivity for the next 10 years.
Despite acknowledging risks associated with glyphosate, the intricate challenge arises from the practicality of replacing such a widely used and efficient herbicide. Agricultural practices, particularly in large-scale farming, heavily rely on glyphosate for weed control due to its affordability and effectiveness. The difficulty lies in finding a suitable alternative that matches its efficiency without significantly increasing costs or compromising agricultural productivity. Moreover, the economic ramifications of banning glyphosate are significant. Farmers, especially smaller ones with limited resources, may face challenges in adopting alternative methods or products. This poses a risk of decreased productivity and potentially higher food prices for consumers.
Therefore, the decision-making process on the future of glyphosate becomes highly complex. Balancing the health and environmental risks associated with glyphosate against its economic impact on agriculture demands a nuanced approach. Striking this balance between the development of sustainable strategies that simultaneously ensure agricultural productivity and safeguard human health and the environment. The conflicting factors of health concerns, environmental impact, economic feasibility, and agricultural efficiency create a challenging landscape for policymakers aiming to address the glyphosate issue within the framework of the precautionary principle.